It's been several decades but the mnemonics still work - Prof. Honegger taught us that when it comes to interstitials in iron, there's a BONCH of them. As in B, O, N, C, H. It may well be that the B-O was to represent only Boron. I do not recall if there was a specific discussion of these elements' effective atomic radius within the metallic matrix but they were taught to us as non-substitutional. In hindsight, oxygen is very reactive to be considered an atom that will migrate, but its calculated atomic radius is comparable to or even smaller than those other elements in this list.
Original Message:
Sent: 03-10-2024 10:44
From: John Grubb
Subject: Relative size of carbon and iron atoms
If I were going to change "interstices", I'd pick "interstitial spaces".
Anything can be an interstitial atom. Irradiated materials have many self interstitials. They're high energy and quickly diffuse to regions (like grain boundaries) where they can better be accommodated. Relative size determines the likelihood of any atom being interstitial or substitutional.
I wouldn't get too attached to sizes that are determined for isolated atoms. We're interested here in atoms embedded in metallic crystals. How they interact with the "electron gas" that permeates the crystal is critical. If they contribute electrons (and shrink) or attract electrons (and swell) will control how they behave.
--
Original Message:
Sent: 3/9/2024 1:39:00 PM
From: Benjamin Huebner
Subject: RE: Relative size of carbon and iron atoms
Out of curiosity, what is more meaningful about saying "interstitial regions" rather than "interstices"?
It is also interesting that even the simplest Google search immediately yields disparate information for the radius of a carbon atom (even if in the same magnitude), shown below:
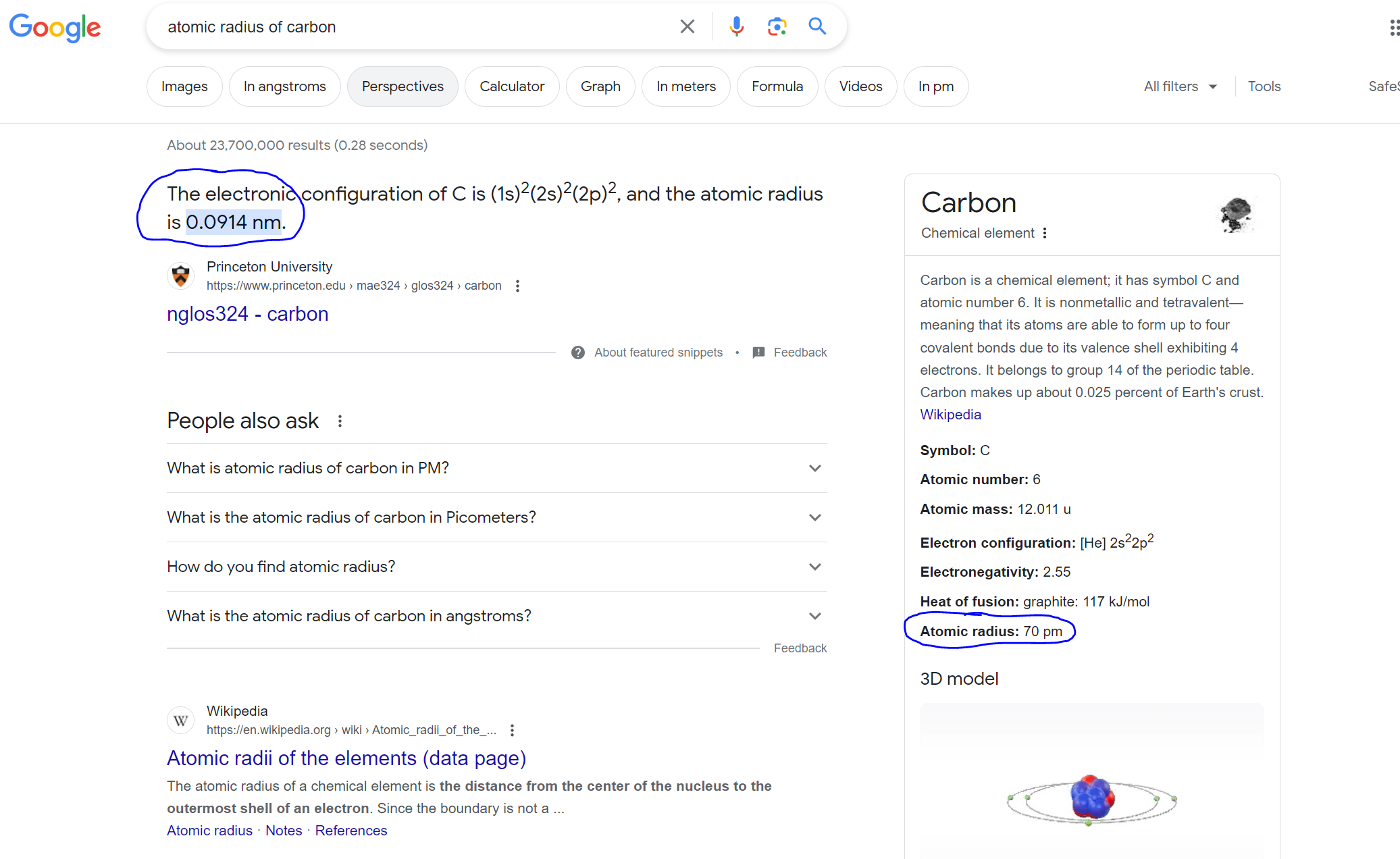
Secondly, are hydrogen, nitrogen, and boron the only other elements (or the most common?) that can fit in the interstices/interstitial areas? If not, I assume there can only be a handful of other elements (perhaps a faulty assumption), so why not list the rest of them as well? It is a separate question of whether it is feasible for, let's say, a helium atom to sit inside an interstitial space of an iron matrix.
Curiously, this government database of van der Waals radii lists a carbon atom radius of 170 pm vs. an iron atom radius of 194. It even lists the atomic radius of boron as 192 pm, which seems bizarre from a materials standpoint and makes it appear that boron would more easily be a substitutional element rather than an interstitial one.
------------------------------
Benjamin Huebner
Materials Engineer
US Air Force
Macon GA
6786415792
Original Message:
Sent: 03-07-2024 14:05
From: Scott Henry
Subject: Relative size of carbon and iron atoms
Here's an updated version, incorporating very helpful feedback from @Walter Sperko, @David Coulston, and @Nassos Lazaridis. I removed the bold because some minor changes were made to other sentences in the paragraph.
The responsiveness of steel from heat treatment is due to some important properties of iron and the metallurgical effects of carbon in iron. Fundamentally, all steels are mixtures or, more properly, alloys of iron with a small amount of carbon (along with varying amounts of other alloying elements such as manganese, silicon, chromium, nickel, and molybdenum). One important factor is the relative size of carbon and iron atoms. Carbon atoms are small enough to fit into the interstitial regions of the larger iron atoms, especially in the temperature range where body-centered cubic alpha iron undergoes a phase transformation to face-centered cubic gamma iron. Other atoms small enough to fit in the interstitial regions of iron atoms are hydrogen, nitrogen, and boron. In general, interstitial atoms can easily diffuse--jumping from one interstitial site to another--unlike larger atoms (which can only jump by "substitution" into the vacancies within a crystal lattice). This, along with the effect of temperature on diffusion, makes carbon atoms very mobile during solid-state heating.
Additional feedback is welcome. Thanks all!
------------------------------
Scott Henry
Director of Content and Publishing
ASM International
Original Message:
Sent: 03-06-2024 14:01
From: Walter Sperko
Subject: Relative size of carbon and iron atoms
Hi, Scott,
I suggest that you follow the wording in the next sentence.
One important factor is the relative sizes of carbon and iron atoms. Carbon atoms are small enough to fit in the interstitial regions of the larger iron atoms. Other atoms small enough to fit in the interstitial regions of solid iron atoms are hydrogen, nitrogen, and boron.
Walt
------------------------------
Walter Sperko
President
Sperko Engineering Services Incorporated
Greensboro NC
(336) 674-0600
Original Message:
Sent: 03-05-2024 15:17
From: Scott Henry
Subject: Relative size of carbon and iron atoms
Thanks to everyone who provided responses. The general consensus is that "1/30" is wrong, but a specific ratio is difficult to calculate. Following is the adjusted wording we propose to use (the affected sentences are in bold). I am happy to receive additional feedback about this.
The responsiveness of steel from heat treatment is due to some important properties of iron and the metallurgical effects of carbon in iron. Fundamentally, all steels are mixtures or, more properly, alloys of iron with a small amount of carbon (along with varying amounts of other alloying elements such as manganese, chromium, nickel, and molybdenum). One important factor is the relative size of carbon atoms and iron atoms. Carbon atoms are sufficiently small to fit between the interstices of the larger iron atoms. Other atoms small enough to fit in the interstitial regions of solid iron are hydrogen, nitrogen, and boron. In general, interstitial atoms can easily diffuse-jumping from one interstitial site to another-unlike larger atoms (which can only jump by "substitution" into the vacancies within a crystal lattice). This, along with the effect of temperature on diffusion, makes the mobility of carbon responsive during solid-state heating.
------------------------------
Scott Henry
Director of Content and Publishing
ASM International
Original Message:
Sent: 02-23-2024 11:08
From: Scott Henry
Subject: Relative size of carbon and iron atoms
Several ASM publications (and non-ASM sources) contain information similar to the following paragraph (bold added):
The responsiveness of steel from heat treatment is due to some important properties of iron and the metallurgical effects of carbon in iron. Fundamentally, all steels are mixtures or, more properly, alloys of iron with a small amount of carbon (along with varying amounts of other alloying elements such as manganese, chromium, nickel, and molybdenum). One important effect is the size of carbon atoms relative to that of iron atoms. The carbon atom is only 1/30 the size of the iron atom, and carbon atoms are sufficiently small to fit between the interstices of the larger iron atoms. Other atoms small enough to fit in the interstitial regions of solid iron are hydrogen, nitrogen, and boron. In general, interstitial atoms can easily diffuse-jumping from one interstitial site to another-unlike larger atoms (which can only jump by "substitution" into the vacancies within a crystal lattice). This, along with the effect of temperature on diffusion, makes the mobility of carbon responsive during solid-state heating.
--From Introduction to Steel Heat Treatment, Steel Heat Treating Fundamentals and Processes, Vol 4A, ASM Handbook, ASM International, 2013, p 3–25, https://doi.org/10.31399/asm.hb.v04a.a0005819
A reader recently pointed out that the statement about the relative size of carbon and iron atoms appears to be incorrect. The reader points out, "since the radius of a carbon atom is roughly 70pm and the radius of an iron atom is roughly 140pm, there is no … calculation … which would result in the size difference being a factor of 30." He pointed to the following sources concerning the relative size of atoms:
J. C. Slater; Atomic Radii in Crystals. The Journal of Chemical Physics, 15 November 1964; Vol 41 (No. 10): p 3199–3204. https://doi.org/10.1063/1.1725697
E. Clementi, D. L. Raimondi, W. P. Reinhardt; Atomic Screening Constants from SCF Functions. II. Atoms with 37 to 86 Electrons. The Journal of Chemical Physics. 15 August 1967; Vol 47 (No. 4): p 1300–1307. https://doi.org/10.1063/1.1712084
From some limited investigations, it appears that the size differential may be closer to 1/8 than 1/30. We would be interested in feedback from ASM Members-how did the 1/30 comparison originate? If ASM amends the content, what would be an accurate and useful way to address the size difference between carbon and iron atoms?
------------------------------
Scott Henry
Senior Content Engineer
ASM International
------------------------------